
【原文题目】
Key parameters to be optimized in the development and manufacturing of oral solid-dosage forms
作者:Anil Kane
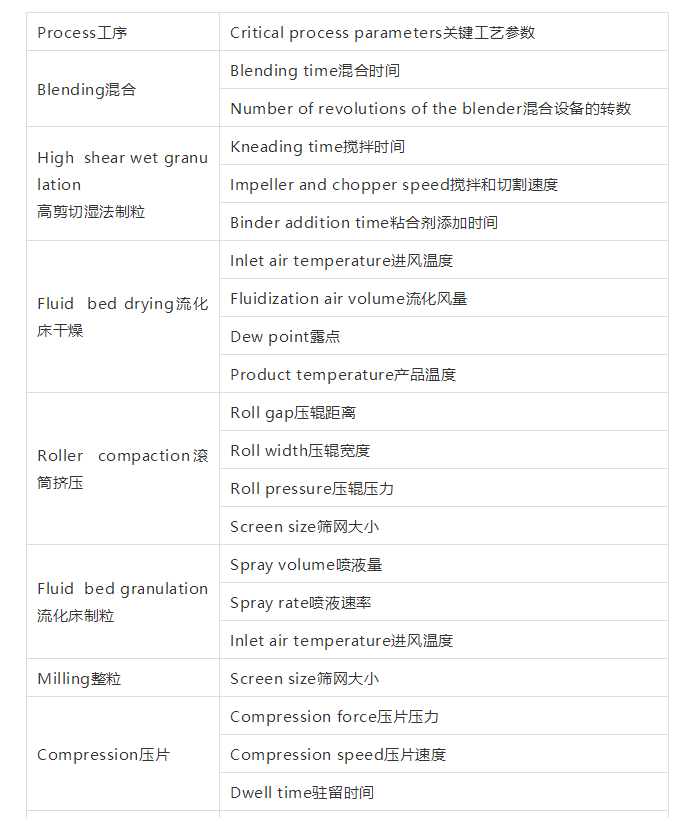
Oral solid-dosage forms (typically, tablets and capsules) are manufactured using conventional manufacturing processes such as direct blending or granulation techniqueswet granulation, dry granulation/roller compaction, or fluid bed granulation followed by fluid bed drying, milling, compression, and coating. It is important to understand the critical processing parameters (CPPs) that impact critical quality attributes(CQA). The CQAs that impact the final outcome (i.e., the therapeutic efficacy of the drug product) usually are parameters such as in-vitro dissolution and drug product stability. In-vitro dissolution of the drug can influence the rate and extent of drug absorption. Drug substance properties such as particle size, morphology, dosage strength, and drug loading have a big impact on the blending process and can influence the homogeneity of the drug substance in the blend. Blending is a critical process that needs to be optimized and is considered a CPP that can impact the potency of the finished product. Similarly, each of the granulation techniques has a processing step that could cause variability and has the potential of impacting the CQAs. Key parameters that need to be optimized (see Table I) will depend on the API properties, its stability, dose, drug loading, and other factors.
口服固体制剂(例如片剂和胶囊剂)都可以采用传统的制造工艺如直接混合造粒技术、湿法造粒技术、干法造粒技术、流化床造粒/干燥技术,包括粉碎、压片、包衣等工序。理解影响关键质量属性(CQA)的关键工艺参数(CPPS)很重要。CQA是那些影响药物最终结果(如疗效)的因素,如体外溶出和药物的稳定性。药物的体外溶出可以影响药物吸收的速度和程度。药物的性质如粒径大小、形态、剂量和载药量对混合过程有很大影响,并会影响药物的混合均匀性。混合是一个需要进行优化的关键,它可以影响成品的含量。每一种制粒技术都包括一个可能引起变异和对CQAs有潜在影响的工序。表1中列出了需要优化的关键参数,这取决于原料药(API)的性质、稳定性、剂量、载药量及其他因素。
Table I: Process and critical process parameters that require optimization.
表1. 需要优化的工序和关键工艺参数

A typical process optimization methodology includes a systematic identification of CQAs, CPPs, and a risk assessment to identify the critical selection of key parameters. A plan can then be drawn up, using a statistical design of experiments (DOE), which can be a full- or partial-factorial design, based on the availability of API and the number of runs that can be manufactured. The DOE experiments involve running parameters at high, medium, and low values, as well as running a defined number of control runs at target parameters. By analyzing the data from the DOE runs, a design space can be identified. Knowledge of the design space enables a control strategy to be defined for the manufacture of the product.
一个典型的工艺优化方法包括系统辨识CQAs、CPPs、对关键参数进行风险评估。然后起草一个方案,可以采用实验设计(DOE)进行一个完全或部分析因设计,这要基于原料药(API)的可获得性和可以生产使用的次数。DOE包括在高、中、低值中进行试验,并在目标参数上进行一定数量的控制试验。通过对DOE数据的分析,可确定设计空间。对设计空间的了解后,则可以对产品的生产制定一个控制策略。
A similar approach is taken for formulation optimization, in which critical functional formulation excipients are evaluated with high, medium, and low levels, and the impact of these variables is studied on output parameters of that functionality (e.g., disintegrant levels studies can be done with the rate of disintegration of tablets as the output).
类似方法还可以进行制剂处方的优化,包括对关键功能性辅料进行高、中、低水平的评价,并通过对输出参数的研究来评估这些变量的影响(例如,考察崩解剂的量可以通过对片剂的崩解情况作为输出变量来研究)。
A systematic DOE to optimize the formulation composition in an oralsolid-dosage form is important for two main purposes:
一个系统的DOE可以优化口服固体制剂的处方组成,其中两个重要的目的是:
(1)To ensure the optimum level of a functional excipient in the formulation. In an immediate-release tablet formulation, excipient levels of binder, disintegrant, and lubricant can be varied and the functionality challenged. A partial or full factorial DOE can evaluate multiple excipients at different levels of the excipient. For an extended-release tablet formulation based on a hydrophilicgel matrix, polymer levels will affect the release rate of the drug from the tablet. Hence, the level of polymer is selected based on the specifications or the target release profile.
(1)确定处方中功能性辅料的最佳用量。在一个速释片剂中,粘合剂、崩解剂、润滑剂的用量和功能是可变的。部分或全因子DOE可在不同水平上评估多种辅料。对于基于亲水性凝胶基质的缓释片剂,聚合物水平会影响片剂的释放速率,因此,聚合物的水平是根据质量标准或目标释放曲线来选择的。
(2)To optimize the drug load in a formulation to reduce the pill burden, especially for a large dose drug product. Many oral solid-dose products need tobe formulated in doses above 100 mg per unit tablet or capsule. There are quite a few new chemical entities that need a much higher dose up to a 500 or 750 mg per unit dose. Fixed-dose combination therapies are becoming more popular, due to synergistic therapeutic effects and as a lifecycle management strategy. The number of actives and the dose per unit tablet are increasing. Thus, there is a need to formulate such products in a way that the tablet size is reasonable to swallow, especially for geriatric patients. It is important, therefore, to optimize the formulation so that the drug load in a tablet is as high aspossible, which also means that there is little room for inert excipients. Selecting the right functional excipient in the right quantity thus becomes more crucial.
(2)优化处方中的载药量以减少服药负担,尤其是大剂量药物。许多口服固体制剂产品(片或胶囊)的规格大于100mg。有相当多的新化合物,需更更高规格如500mg或750mg。由于协同治疗效果且作为一个生命周期管理策略,固定剂量联合疗法正变得越来越流行。这种治疗的治疗次数和服用数量正在增加,因此,有必要制定这样的产品,其片剂的大小是可以合理吞咽的,尤其对老年患者。因此,优化处方使片剂的载药量应尽可能高,这也就意味赋形剂的量会用得很少。正确选择功能性辅料从而变得更加重要。
Products with multiple doses use a “dose weight proportional” strategy-using the same formulation composition (drug to excipient ratio) and compressing the larger dose into a larger tablet size. This strategy has the benefit of avoiding a separate stability study as the drug to excipient ratiois the same; however, the disadvantage is that the higher dose becomes a large tablet, which may present “patient compliance” issues because of difficulty in swallowing. The limited time available for developing clinical trial formulations often makes optimization of drug load and tablet size a low priority.
多规格的产品使用“剂量-重量成比例”的策略,即采用相同的处方组成(药物与赋形剂的比例),较大的剂量会压成较大的药片。这种策略有利于避免进行单独的稳定性研究,因为药物与赋形剂的比例是相同的;然而,缺点是高规格药物是一个大药片,病人吞咽困难,这可能会引起“病人依从性”的问题。由于开发临床试验处方的时间是有限的,这常常使药物载药量的研究、片剂大小的研究降低优先级。
During this stage, however, it is important that the choice ofexcipients, level of excipients, the drug load, and reduction of pill size and burden are optimized. Polypharmacy is a huge problem, and patients would benefit greatly if better drug products that are easier to swallow can be developed. Optimizing the formulation at the right stage in the development program by increasing the drug load per unit tablet or capsule, thereby, reducing the pill burden, would be beneficial.
然而,在这一阶段,重要的是辅料的选择,辅料的水平、载药量、降低药片大小和服药负担是需要优化的。多重用药是一个巨大的问题,如果开发的药物使患者更容易服用,那么患者将大大受益。在开发的适当阶段,通过优化处方,增加每单位片剂或胶囊中的载药量,从而减少服药负担将是有益的。
文章来源:Pharmaceutical Technology Vol. 41, No. 4 Pages: 18
声明:本文由药事纵横小编翻译,转载务必注明来源和作者(原文作者和翻译作者),否则一律认定为侵权。
<END>

















































 浙公网安备33011002015279
浙公网安备33011002015279 本网站未发布麻醉药品、精神药品、医疗用毒性药品、放射性药品、戒毒药品和医疗机构制剂的产品信息
本网站未发布麻醉药品、精神药品、医疗用毒性药品、放射性药品、戒毒药品和医疗机构制剂的产品信息
收藏
登录后参与评论
暂无评论