
近日,复宏汉霖针对创新型抗HER2单抗HLX22作用机制的研究结果刊登于医学领域知名期刊Journal of Translational Medicine。该研究分析和验证了HLX22相关的结构基础和作用机制,揭示了其联合曲妥珠单抗在一线治疗HER2阳性胃/胃食管交界部癌患者中的治疗潜力,有望为更广泛的患者群体带来获益。

曲妥珠单抗作为首个靶向HER2的抗肿瘤药物,已广泛应用于HER2阳性乳腺癌和胃癌的临床实践,重塑了这些领域的治疗格局。曲妥珠单抗与帕妥珠单抗及化疗的联用,也被证明存在协同机制,成为HER2 阳性转移性乳腺癌的一线标准疗法。然而,该双靶向疗法却在HER2阳性转移性胃癌患者中取得了阴性结果,提示HER2 阳性晚期乳腺癌及胃癌间存在生物学差异。目前,全球范围内尚未有治疗HER2阳性胃癌的双靶向疗法获批上市。

HLX22为复宏汉霖自AbClon, Inc.许可引进、并后续自主研发的靶向HER2的创新型单克隆抗体。与曲妥珠单抗类似,HLX22可结合在HER2的亚结构域IV,但结合表位与曲妥珠单抗不重叠,不产生竞争性结合。因此,HLX22和曲妥珠单抗联合使用时,两款药物能够同时与HER2结合,从而产生更强的HER2受体阻断效果。体内及体外研究表明,HLX22与曲妥珠单抗(HLX02)联用在人胃癌细胞株及多种胃癌CDX/PDX 模型中都展现出强大的抗肿瘤活性,能够协同增效促进肿瘤细胞的凋亡。
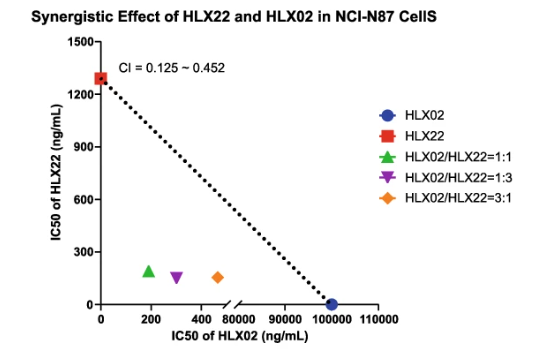


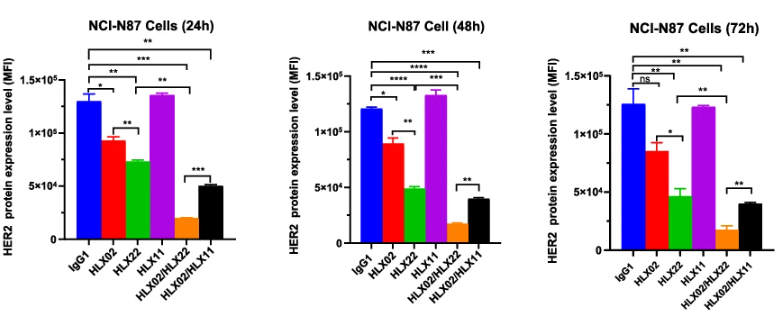
表皮生长因子受体(EGFR)家族在肿瘤发生、转移及化疗耐药性等信号传导中发挥重要作用[1]。人表皮生长因子受体2(HER2)是EGFR家族成员之一,该家族还包括EGFR(HER1)、HER3及HER4。与配体结合时,EGFR家族蛋白能够通过同源二聚化或异源二聚化,形成不同的受体复合物,进而引起胞内结构域上的蛋白磷酸化,激活细胞内信号级联通路,最终影响细胞生长、增殖、迁移和存活的各类生命过程[2, 3]。研究结果显示,当HLX22和曲妥珠单抗(HLX02)共同作用于HER2的亚结构域IV时,可以同时促进 HER2/HER2 同源二聚体和 HER2/EGFR 异源二聚体的内吞作用,将HER2的内吞提高40-80%。相较而言,帕妥珠单抗会抑制HER2的异源二聚化,因此曲妥珠单抗和帕妥珠单抗的联用仅能促进HER2/HER2同源二聚体的内吞,而无法促进HER2/EGFR异源二聚体的内吞。这或许也是导致曲妥珠单抗与帕妥珠单抗这一双靶向疗法未能在HER2阳性胃癌临床研究中取得疗效的原因。


HLX22的 I 期临床研究也表明,HLX22 在晚期HER2阳性胃癌患者中具有良好的耐受性和药代动力学特性,未出现剂量限制性毒性[4],该研究结果已发表于期刊Investigational New Drugs。目前,HLX22联合汉曲优®(曲妥珠单抗,美国商品名:HERCESSI™,欧洲商品名:Zercepac®)及化疗一线治疗HER2阳性局部晚期/转移性胃癌的 II 期临床试验(HLX22-GC-201)正在开展中,其研究数据发布于2024年美国临床肿瘤学会胃肠道肿瘤研讨会(ASCO GI)、Cell Press细胞出版社旗下的综合性医学旗舰期刊MED及2024年欧洲肿瘤学会胃肠道肿瘤研讨会(ESMO GI)。研究结果显示,在HLX02(曲妥珠单抗)联用化疗的基础上加入HLX22可明显改善HER2阳性G/GEJ癌患者一线治疗的效果,且安全性可控。此外,公司就HLX22开展的国际多中心 III 期新药临床试验(IND)申请也相继获得中国国家药品监督管理局(NMPA)和美国食品药品监督管理局(FDA)许可。
未来,期待HLX22与曲妥珠单抗的双靶向疗法能够在临床研究中进一步验证其优异的疗效及安全性,改变HER2阳性胃癌的治疗模式。复宏汉霖也将持续加码创新,以患者为中心,以临床需求为先导,继续高效地为全球患者提供更多可负担、疗效更好的治疗方案。
关于Journal of Translational Medicine
Journal of Translational Medicine是Springer Nature旗下开放获取的医学期刊,旨在发表来自人体实验信息的研究成果,以促进基础科学与临床科学之间的交流与转化。该期刊涵盖转化医学的所有领域,并设置多个专栏。
关于复宏汉霖
复宏汉霖(2696.HK)是一家国际化的创新生物制药公司,致力于为全球患者提供可负担的高品质生物药,产品覆盖肿瘤、自身免疫疾病、眼科疾病等领域,已在中国上市5款产品,在国际获批上市3款产品,23项适应症获批,3个上市申请分别获中国药监局和欧盟EMA受理。自2010年成立以来,复宏汉霖已建成一体化生物制药平台,高效及创新的自主核心能力贯穿研发、生产及商业运营全产业链。公司已建立完善高效的全球创新中心,按照国际药品生产质量管理规范(GMP)标准进行生产和质量管控,不断夯实一体化综合生产平台,其中,公司商业化生产基地已相继获得中国、欧盟和美国GMP认证。
复宏汉霖前瞻性布局了一个多元化、高质量的产品管线,涵盖50多个分子,并全面推进基于自有抗PD-1单抗H药汉斯状®的肿瘤免疫联合疗法。继国内首个生物类似药汉利康®(利妥昔单抗)、自主研发的中美欧三地获批单抗生物类似药汉曲优®(曲妥珠单抗,美国商品名:HERCESSI™,欧洲商品名:Zercepac®)、汉达远®(阿达木单抗)和汉贝泰®(贝伐珠单抗)相继获批上市,创新产品汉斯状®(斯鲁利单抗)已获批用于治疗微卫星高度不稳定(MSI-H)实体瘤、鳞状非小细胞肺癌、广泛期小细胞肺癌和食管鳞状细胞癌,并成为全球首个获批一线治疗小细胞肺癌的抗PD-1单抗。公司亦同步就16个产品在全球范围内开展30多项临床试验,对外授权全面覆盖欧美主流生物药市场和众多新兴市场。
Publication in Journal of Translational Medicine Highlighting Henlius' Dual HER2 Blockade Therapy Mechanism of Action
Recently, Journal of Translational Medicine, a prestigious medical journal, published a research article describing mechanism of action (MOA) of Henlius' innovative anti-HER2 monoclonal antibody (mAb), HLX22, in dual HER2 blockade therapy. The research analysed the structure foundation and mechanisms of action associated with HLX22, further validating its potential in combination with trastuzumab in the first-line treatment of HER2-positive gastric/gastroesophageal junction (G/GEJ) cancer to benefit more patients worldwide.
Trastuzumab, the first HER2-targeted cancer therapy, was introduced in clinical practice and revolutionised the treatment of HER2-positive breast cancer and gastric cancer. Trastuzumab in combination with pertuzumab and docetaxel has also verified their synergistic effect in the treatment of HER2-positive metastatic breast cancer, and the combination regimen is now the standard of care in this indication. However, a phase 3 trial that assessed the efficacy of pertuzumab versus placebo in combination with trastuzumab and chemotherapy in first-line HER2-positive metastatic gastric or G/GEJ cancer showed negative result, indicating intrinsic differences in the tumour biology of HER2-positive advanced gastric cancer and HER2-positive breast cancer. Up to date, no dual HER2 blockade therapy for the treatment of HER2-positive gastric cancer has been approved.
HLX22 is an innovative anti-HER2 mAb that was introduced from AbClon, Inc. and further researched and developed by Henlius. HLX22 can bind to HER2 subdomain IV at a non-overlapping binding site from trastuzumab, which avoids a competitive binding between HLX22 and trastuzumab to HER2 and allows the simultaneous binding, contributing to a synergistic effect of the dual targeting therapy. Both in vitro and in vivo studies demonstrated that HLX22 in combination with trastuzumab (HLX02) exhibit enhanced anti-tumour activity and induced cancer cell apoptosis in HER2-positive human gastric cancer cell lines, gastric cancer CDX models, and PDX models.
Epidermal growth factor receptor (EGFR) family signaling contributes to neoplastic cell growth, malignant transformation, and resistance to chemotherapy[1]. Human epidermal growth factor receptor 2 (HER2) belongs to human EGFR family, including EGFR (HER1), HER3, and HER4. Upon ligand binding, the EGFR family members can homodimerise or heterodimerise with each other to form several receptor complexes. The dimerisation of EGFR family members results in protein phosphorylation on their intracellular domain that activates intracellular signaling cascades, which ultimately promote the expression of target genes that regulate various cellular processes influencing growth, proliferation, migration and survival[2,3]. Remarkably, HLX22 and trastuzumab (HLX02) simultaneously bound to HER2 subdomain IV and promoted HER2/HER2 homodimer internalisation and HER2/EGFR heterodimer internalisation, resulting in a 40%–80% increase in HER2 internalisation. In contrast, pertuzumab disrupted the formation of HER2 heterodimer. Therefore, trastuzumab (HLX02) and HLX11(pertuzumab biosimilar) combination disrupted HER2/EGFR heterodimer formation. Consequently, trastuzumab (HLX02) and HLX11(pertuzumab biosimilar) combination only promoted HER2 internalisation but could not promote EGFR internalisation together. This might be one of the reasons why trastuzumab and pertuzumab combination failed in gastric cancer clinical trial.
Results of the phase 1 clinical trial, published in Investigational New Drugs, also showed that HLX22 was well tolerated with favorable pharmacokinetic properties in patients with advanced HER2 overexpressing solid tumours, and no dose-limiting toxicity occurred during the study [4]. As of now, the phase 2 study of HLX22 (HLX22-GC-201) is ongoing and its results were released at the 2024 ASCO Gastrointestinal Cancers Symposium (ASCO GI), MED, a flagship medical journal published monthly by Cell Press, and the 2024 ESMO Gastrointestinal Cancers Congress (ESMO GI). The results showed that add HLX22 to trastuzumab (HLX02) and chemotherapy improved efficacy in patients with HER2-positive G/GEJ cancer in the first-line setting, with a manageable safety profile. Recently, the investigational new drug application (IND) for phase 3 international multicentre clinical study of HLX22 in combination with trastuzumab and chemotherapy for the first-line treatment of HER2-positive advanced gastric cancer has been approved by the National Medical Products Administration (NMPA) and the United States Food and Drug Administration (FDA).
Looking forward, the company will further validate the efficacy and safety of the dual HER2 blockade therapy of HLX22 in clinical trials, reshaping the paradigm for treatment of HER2-positive gastric cancer. Meanwhile, the company will actively improve efficiency through innovations, focusing on patients and unmet medical needs to bring more high-quality and affordable therapies to patients worldwide.
About Journal of Translational Medicine
Journal of Translational Medicine is an open access journal publishing articles focusing on information derived from human experimentation so as to optimise the communication between basic and clinical science. The journal covers all areas of translational medicine and has several specialized sections.
About Henlius
Henlius (2696.HK) is a global biopharmaceutical company with the vision to offer high-quality, affordable, and innovative biologic medicines for patients worldwide with a focus on oncology, autoimmune diseases, and ophthalmic diseases. Up to date, 5 products have been launched in China, 3 have been approved for marketing in overseas markets, 23 indications are approved worldwide, and 3 marketing applications have been accepted for review in China and the EU, respectively. Since its inception in 2010, Henlius has built an integrated biopharmaceutical platform with core capabilities of high-efficiency and innovation embedded throughout the whole product life cycle including R&D, manufacturing and commercialization. It has established global innovation centre and Shanghai-based commercial manufacturing facilities certificated by China, the EU and U.S. GMP.
Henlius has pro-actively built a diversified and high-quality product pipeline covering over 50 molecules and has continued to explore immuno-oncology combination therapies with proprietary HANSIZHUANG (anti-PD-1 mAb) as backbone. Apart from the launched products HANLIKANG (rituximab), the first China-developed biosimilar, HANQUYOU (trastuzumab, trade name: HERCESSI™ in the U.S., Zercepac® in Europe), a China-developed mAb biosimilar approved in China, Europe and U.S., HANDAYUAN (adalimumab) and HANBEITAI (bevacizumab), the innovative product HANSIZHUANG has been approved by the NMPA for the treatment of MSI-H solid tumours, squamous non-small cell lung cancer (sqNSCLC) and extensive-stage small cell lung cancer (ES-SCLC), and esophageal squamous cell carcinoma (ESCC), making it the world’s first anti-PD-1 mAb for the first-line treatment of SCLC. What’s more, Henlius has conducted over 30 clinical studies for 16 products, expanding its presence in major markets as well as emerging markets.





































 浙公网安备33011002015279
浙公网安备33011002015279 本网站未发布麻醉药品、精神药品、医疗用毒性药品、放射性药品、戒毒药品和医疗机构制剂的产品信息
本网站未发布麻醉药品、精神药品、医疗用毒性药品、放射性药品、戒毒药品和医疗机构制剂的产品信息
收藏
登录后参与评论