2024年9月9日,复宏汉霖(2696.HK)宣布,公司自主研发的创新型单抗H药 汉斯状®(斯鲁利单抗)小细胞肺癌领域最新研究结果以壁报形式在2024年世界肺癌大会(World Conference on Lung Cancer, WCLC)上发布。
H药 汉斯状®(斯鲁利单抗)是全球首个一线治疗小细胞肺癌的的抗PD-1单抗,已在中国、印尼、柬埔寨、泰国等地获批上市。目前,H药在中国已获批用于治疗微卫星高度不稳定(MSI-H)实体瘤、鳞状非小细胞肺癌(sqNSCLC)、广泛期小细胞肺癌(ES-SCLC)及食管鳞状细胞癌(ESCC),覆盖肺癌、消化道肿瘤等高发肿瘤,累计惠及约8万名患者。
肺癌是全球最常见的恶性肿瘤之一。其中SCLC约占肺癌总数的15%[1],是肺癌中侵袭性最强的亚型,分为局限期小细胞肺癌(LS-SCLC)和ES-SCLC。截至目前,H药一线治疗ES-SCLC的适应症已在中国、印尼、柬埔寨和泰国获批,其欧盟上市许可申请(MAA)也已获得欧洲药品管理局 (EMA) 受理,有望于今年获批上市。同时,公司围绕H药在肺癌一线治疗全面布局,开展了针对sqNSCLC、ES-SCLC和LS-SCLC的多项国际多中心III期临床试验,并在美国启动了一项H药对比一线标准治疗阿替利珠单抗用于ES-SCLC的头对头桥接试验。
此次2024 WCLC大会的H药研究数据结果如下:
ASTRUM-005R研究

论文题目
ASTRUM-005R:斯鲁利单抗联合化疗一线治疗广泛期小细胞肺癌的真实世界多中心研究

试验设计
这是一项评估斯鲁利单抗联合化疗一线治疗ES-SCLC疗效和安全性的多中心、真实世界队列研究。纳入2022年4月至2024年4月在中国14家研究中心接受至少两个周期斯鲁利单抗联合依托泊苷和铂类一线治疗的ES-SCLC患者,并且至少进行一次疗效评估,主要研究终点为真实世界无进展生存期(rwPFS),次要终点包括总生存期(OS)、客观缓解率(ORR)、安全性。

结果
研究共入组538例患者进行疗效和安全性分析。中位年龄为63(范围:57.00~68.75)岁,467例(86.80%)为男性患者,406例(75.46%)患者有吸烟史。ECOG PS 为 0 或 1患者占多数(491,91.26%)。排除其他原发性肿瘤外,237例(44.05%)伴有合并症。肝转移和脑转移发生率分别为33.09%和21.56%。免疫联合化疗的中位治疗周期数为4(范围:4~6)周期,222例(41.26%)患者接受了超过4周期的治疗。
疗效数据显示,中位 rwPFS 为 9.1 个月(95% CI:8.1~9.7),1 年 rwPFS 率为 34.6%,超过 ASTRUM-005 研究亚裔人群的 1 年 PFS 率 28.2%,2 年 rwPFS 率为 11.3%。无肝转移患者的中位 rwPFS 为 9.7 个月(95%CI:8.8~12.8)显著优于肝转移患者的 7.4 个月(95%CI:6.2~8.9)(P < 0.0001),接受 >4 周期免疫联合化疗较接受 ≤4 周期免疫联合化疗治疗的患者中位 rwPFS显著提高( 10.5 个月(95%CI:9.3~13.3)vs 6.7 个月(95% CI: 6.3~9.1)(P< 0.0001))。ORR为71.29%,OS尚未成熟。安全性方面,AESI发生率为38.85%,其中≥3级AESI为15.43%。

结论
斯鲁利单抗联合化疗一线治疗ES-SCLC的真实世界生存结果,为临床实践提供有价值的见解,验证了ASTRUM-005研究结果,为斯鲁利单抗联合依托泊苷和铂类一线治疗ES-SCLC补充有力证据。
一项回顾性研究

论文题目
免疫联合化疗对比化疗新辅助治疗局限期小细胞肺癌:一项回顾性研究

试验设计
研究纳入2018年1月至2023年10月接受新辅助PD-L1/PD-1抑制剂联合铂类化疗或单纯铂类化疗后进行手术切除的LS-SCLC患者。主要研究终点为病理完全缓解(pCR)和主要病理缓解(MPR)。次要研究终点包括R0切除率、降期率和无病生存期(DFS)。

结果
研究入组51例患者,其中26例接受免疫联合化疗新辅助治疗(14例(53.8%)接受斯鲁利单抗联合化疗新辅助治疗),25例接受化疗新辅助治疗,男性和女性患者分别有39名和12名。免疫联合化疗组和化疗组的平均年龄分别为 61.6(43.1~74.5)岁和 59.5(45.8~73.2)岁(表1)。
疗效数据显示,免疫联合化疗组和化疗组的pCR分别为 38.5%和 8.0%(OR 7.2,95% CI:1.4~37.3)。免疫联合化疗组和化疗组的MPR分别为 53.8%和12.0%(OR 8.6,95% CI:2.0~35.8)。免疫联合化疗组和化疗组R0切除率分别为92.3%和80.0%(OR 3.0,95% CI:0.5~17.2)。免疫联合化疗组较化疗组的病理降期率(88.5% vs 36.0%)和N2降期率(68.2% vs 24.0%)均提升(表2)。中位随访时间为17.2个月,免疫联合化疗组的中位DFS尚未达到,化疗组的中位DFS为11.3(95% CI:6.1~16.5)个月。

表1 患者基线特征
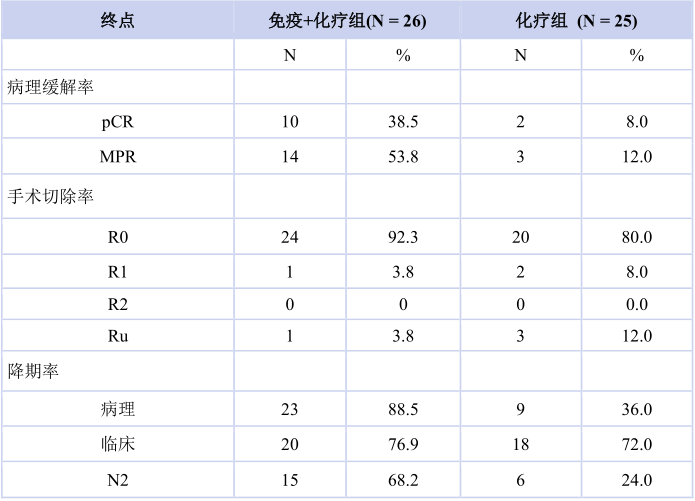
表2 研究结果

图1 不同新辅助治疗组的无病生存期结果

结论
新辅助免疫检查点抑制剂联合化疗治疗LS-SCLC显示出良好的疗效和可行性,可作为LS-SCLC潜在治疗选择,未来可进一步开展前瞻性随机对照试验证明免疫联合化疗新辅助治疗LS-SCLC的疗效与安全性,以优化LS-SCLC的治疗策略。
【参考文献】
[1] Eskandar A, Ahmed A, Daughtey M, et al. Racial and sex differences in presentation and outcomes of small cell lung cancer in the United States: 1973 to 2010[J].Chest, 2015,147(4): e164-e165.
关于H药 汉斯状®
关于复宏汉霖
Latest Results of Serplulimab in the Field of SCLC Released at 2024 WCLC
Shanghai, China, September 9, 2024 – Shanghai Henlius Biotech, Inc. (2696.HK) announced that the latest results of the company’s self-developed innovative anti-PD-1 monoclonal antibody (mAb), HANSIZHUANG (serplulimab) were released as poster presentations at 2024 World Conference on Lung Cancer (“WCLC 2024”) in the field of small cell lung cancer (SCLC).
HANSIZHUANG, the world's first anti-PD-1 mAb approved for the first-line treatment of SCLC, has been launched in China, Indonesia, Cambodia and Thailand. Up to date, it has been approved by the National Medical Products Administration (NMPA) for the treatment of MSI-H solid tumours, squamous non-small cell lung cancer (sqNSCLC), extensive-stage small cell lung cancer (ES-SCLC), and esophageal squamous cell carcinoma (ESCC), broadly covering high-incidence tumours including lung cancer and gastrointestinal cancer, benefiting about 80,000 patients.
Lung cancer is one of the most common malignancies around the world. SCLC is the most aggressive subtype of lung cancer, accounting for around 15% of all lung cancer cases[1]. The SCLC breaks down into limited stage small cell lung cancer (LS-SCLC) and ES-SCLC. To date, HANSIZHUANG has been approved for the first-line treatment of ES-SCLC in China, Indonesia, Cambodia and Thailand. Its Marketing Authorisation Application (MAA) for ES-SCLC has been validated by the European Medicines Agency (EMA), which is expected to be approved in 2024. In the meantime, Henlius has successively conducted a number of immuno-combination therapy clinical trials with serplulimab, including several global multi-centre phase 3 clinical trials regarding sqNSCLC, ES-SCLC and LS-SCLC, covering the full range of first-line treatments of lung cancer. Henlius has also initiated a head-to-head bridging trial in the United States to compare HANSIZHUANG to standard of care atezolizumab (anti-PD-L1 mAb) for the first-line treatment of ES-SCLC.
The latest data of the studies released at 2024 WCLC conference are as follows:
ASTRUM-005R

Title
Real-world First-line Serplulimab-based Immunochemotherapy for Extensive-stage Small Cell Lung Cancer: The Multicenter ASTRUM-005R Study

Study design
This cohort study enrolled adult patients with ES-SCLC at 14 institutions in China from April 2022 to April 2024. These patients received at least two cycles of first-line serplulimab combined with chemotherapy and underwent at least one response assessment. The primary endpoint was the investigator-assessed real-world progression-free survival (rwPFS) according to RECIST version 1.1. Secondary endpoints included overall survival (OS), objective response rate (ORR), and safety profiles refer to CTCAE version 5.0.

Results
A total of 538 patients were enrolled and evaluated for both efficacy and safety. The participants had a median age of 63 years (range 57.00-68.75), with 467 (86.80%) being male and 406 (75.46%) having a history of smoking. Most patients (491, 91.26%) had an ECOG PS of 0 or 1. Comorbidities, excluding other primary tumors, were present in 44.05% (237 patients). Liver and brain metastases were observed in 33.09% (n=178) and 21.56% (n=116), respectively. The median number of immunochemotherapy cycles was 4 (range 4-6), with 41.26% (n=222) receiving more than four cycles. The median rwPFS was 9.1 months (95% CI 8.1-9.7), with a 1-year rwPFS rate of 34.6%, surpassing the 1-year PFS rate of 28.2% reported for the Asian in the ASTRUM-005 study. Besides, the 2-year rwPFS rate was shown to be 11.3%. The median rwPFS was 9.7 months (95% CI 8.8-12.8) in patients without liver metastasis, significantly longer than the 7.4 months (95% CI 6.2-8.9) in patients with liver metastasis (P < 0.0001). Patients who received >4 cycles of immunochemotherapy had a median rwPFS of 10.5 months (95% CI 9.3-13.3), which was significantly longer than the 6.7 months (95% CI 6.3-9.1) in patients who received ≤4 cycles (P < 0.0001). The OS was not yet mature, and the ORR was 71.29%. The occurrence frequency of adverse event of special interest (AESI) was 38.85% (421 events), in which grade ≥3 AESIs occupied 15.43%.

Conclusion
The ASTRUM-005R study provides additional empirical evidence to support the therapeutic value of serplulimab plus chemotherapy and complements the pivotal data from the ASTRUM-005 clinical trial.
A retrospective study

Title
Is neoadjuvant chemoimmunotherapy better than chemotherapy in patients with limited-stage small cell lung cancer: a retrospective comparative study

Study design
Patients with LS-SCLC who received neoadjuvant PD-L1/PD-1 inhibitor plus platinum-based chemotherapy or platinum-based chemotherapy alone followed by surgery between Jan 2018 and Oct 2023 in our institute were included. The primary endpoints were pathologic complete response rate and major pathologic response. The second endpoints were R0 resection rate, downstaging rate and disease-free survival.

Results
Of the 51 eligible patients, 26 received neoadjuvant chemoimmunotherapy and 25 received neoadjuvant chemotherapy (Table 1). There were 39 males and 12 females across both groups combined. Mean (range) age at diagnosis was 61.6 years (43.1-74.5) for the chemoimmunotherapy group and 59.5 years (45.8-73.2) for chemotherapy group. Pathologic complete response rate was 38.5% (10/26) in chemoimmunotherapy group versus 8.0% (2/25) in chemotherapy group (OR 7.2, 95% CI 1.4-37.3) (Table 2). Major pathologic response rate was 53.8% (14/26) in chemoimmunotherapy group versus 12.0% (3/25) in chemotherapy group (OR 8.6, 95% CI 2.0-35.8). R0 resection rate was 92.3 (24/26) in chemoimmunotherapy group versus 80.0% (20/25) in chemotherapy group (OR 3.0, 95% CI 0.5-17.2). Pathologic downstaging rate (88.5% vs 36.0) and N2 downstaging rate (68.2 vs 24.0) were higher in chemoimmunotherapy group. After a median follow up of 17.2 months, median DFS was not reached in chemoimmunotherapy group versus 11.3 (95% CI, 6.1-16.5) months in chemotherapy group.

Conclusion
Neoadjuvant immune checkpoint inhibitors (ICIs) combined with chemotherapy demonstrated promising efficacy and feasibility, and are a potential strategy for the management of LS-SCLC. Further prospective randomized controlled trials are necessary to clearly define the benefit of neoadjuvant chemoimmunotherapy and optimize LS-SCLC treatment.
About HANSIZHUANG
About Henlius
联系方式
媒体:PR@Henlius.com
投资者:IR@Henlius.com







喜欢本文内容
点击下方按钮·分享 ·收藏 ·点赞 ·在看



































 浙公网安备33011002015279
浙公网安备33011002015279 本网站未发布麻醉药品、精神药品、医疗用毒性药品、放射性药品、戒毒药品和医疗机构制剂的产品信息
本网站未发布麻醉药品、精神药品、医疗用毒性药品、放射性药品、戒毒药品和医疗机构制剂的产品信息
收藏
登录后参与评论